Valence Electrons in Br
The total number of electrons in the valence shell of each atom remains constant in this reaction. That is the Group 7A.
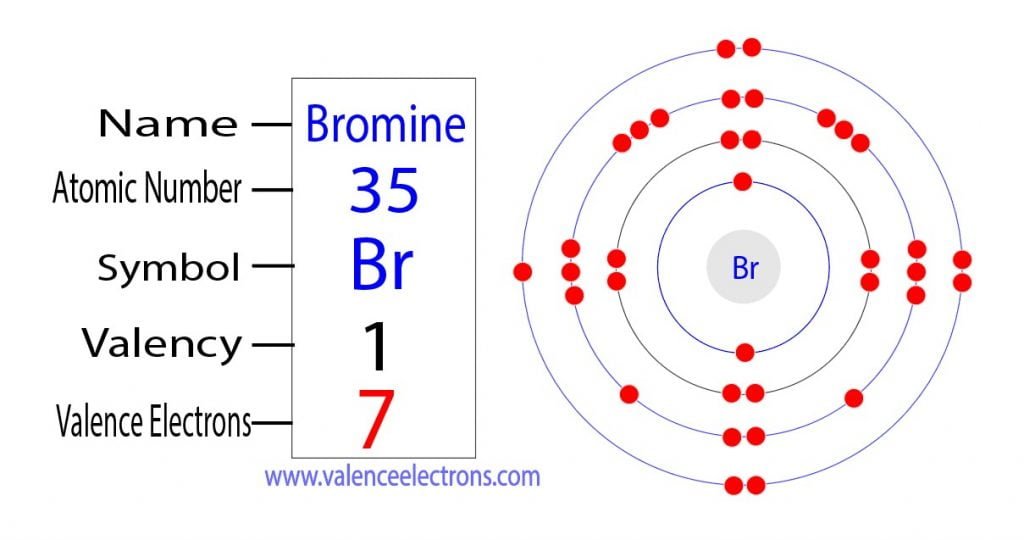
How Many Valence Electrons Does Bromine Br Have
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.

. For example to assume that aluminum bromide contains Al 3 and Br-ions. 35 Br Bromine 79904. 39 Y Yttrium 88906.
Dans un ion monoatomique sa valence est sa charge on parle alors délectrovalence. 40 Zr Zirconium 91224 2 8 18 10 2. We can examine trends in ionic radii across a row of the periodic table by comparing data for atoms and ions that are isoelectronic atoms or ions that have the same number of electrons.
Br Bromine 79904 2 8 18 7. If you look at the periodic table and at the period numbers that is the number of valence electrons. After electrons were discovered chemists became convinced that oxidation-reduction reactions involved the transfer of electrons from one atom to another.
39 Y Yttrium 88906 2 8 18 9 2. One loosely bound valence electron. Find your element on the table.
Electronic Structure Test Questions. You can do this with its chemical symbol the letters in each box its atomic number the number in the top left of each box or any of the other pieces of information available to you on the table. These elements have also been referred to as the triels.
For example the s sublevel can only hold two electrons so the 1s is filled at helium 1s 2. Fluorine is a halogen element and its symbol is F. Valence Electron Definition in Chemistry.
Valence electrons are the electrons contained in the outermost shell. If the number is larger than 10 subtract 10 so you get two valence electrons. Readily form divalent cations.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. La valence dun élément chimique est le nombre maximal de liaisons covalentes ou ioniques quil peut former en fonction de sa configuration électroniqueDans une molécule ou un ion la valence dun atome est le nombre de liaisons covalentes que cet atome a formées. 40 Zr Zirconium 91224.
There are more elements in some periods than others because the number of elements is determined by the number of electrons allowed in each energy sub-level. 43 Tc Technetium 98907 2 8 18 13 2. Many elements have a common valence related to their position in the periodic table and nowadays this is rationalised by the octet ruleThe GreekLatin numeral prefixes mono-uni- di-bi- tri-ter- and so on are used to describe ions in the charge states 1 2 3 and so on respectively.
38 Sr Strontium 8762. Noble Gas Core Definition. Other electronegativity scales include the Mulliken scale proposed by Robert S.
Principal Energy Level Definition. For example purposes lets find the valence. 41 Nb Niobium 92906 2 8 18 12 2.
If we subtract 10 from 16 we get 6. The boron group are the chemical elements in group 13 of the periodic table comprising boron B aluminium Al gallium Ga indium In thallium Tl and perhaps also the chemically uncharacterized nihonium Nh. Oxygen is in the 16th period.
The nonmetals gain electrons until they have the same number of electrons as the nearest noble gas Group 8A forming negatively charged anions which have charges that are the group number minus eight. 38 Sr Strontium 87620 2 8 18 8 2. Highly reactive with reactivity increasing moving down the group.
Two electrons in the valence shell. 42 Mo Molybdenum 9595 2 8 18 13 1. Periodic Table of Elements.
Visualize trends 3D orbitals isotopes and mix compounds. 36 Kr Krypton 83798. Elements in a group share a common number of valence electrons.
Mulliken in 1934 in which the first ionization energy and electron affinity are averaged together and the Allred-Rochow scale which measures the electrostatic attraction between the nucleus of an atom and its valence electrons. Interactive periodic table showing names electrons and oxidation states. The elements in the boron group are characterized by having three valence electrons.
Fluorine is the 9th element in the periodic table and the first element in group-17. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Polyvalence or multivalence refers to species that are not restricted to a specific.
37 Rb Rubidium 84468 2 8 18 8 1. 41 Nb Niobium. 37 Rb Rubidium 85468.
The lanthanides rare earth and actinides are also transition metals. Now locate the element that you want to find the valence electrons for on the table. The table below summarizes data on the radii of a series of isoelectronic ions and atoms of second- and.
Patterns in Ionic Radii. Atoms become larger as we go down a column of the periodic table. 36 Kr Krypton 83768 2 8 18 8.
They also have a larger number of valence electrons and are already close to having a complete octet of eight electrons. K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr first period with d-block elements. Fluorine participates in the formation of bonds through valence electronsThis article discusses in detail how to easily calculate the number of valence electrons in fluorine.
44 Ru Ruthenium 10107 2 8 18 15 1.
Chem4kids Com Bromine Orbital And Bonding Info

How Many Valence Electrons Does Bromine Br Have Valency Of Bromine

How Many Valence Electrons Does Bromine Have Youtube
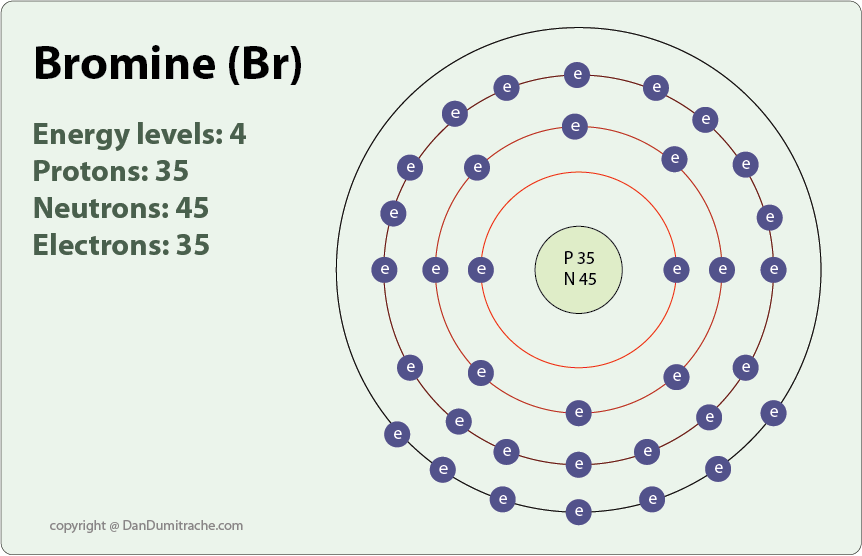
Why Does Fluorine Have A Higher Ionization Energy Than Bromine Socratic
No comments for "Valence Electrons in Br"
Post a Comment